 What’s the deal with the universe? That’s a question comedian Jerry Seinfeld might well posit in one of his stand-up performances. It came from nothing, it goes back to nothing and in between the coming and going there’s a whole lot of nothing. Seinfeld became immortalized by the eponymous sitcom that famously billed itself as “a show about nothing.” Of course, from that show about nothing sprang a cornucopia of expressions that have become part of the lingua franca – re-gifter, sideler, close-talker, man-hands, master-of-my-own-domain, yada, yada yada. And out of the nothingness from which the universe sprang we got – well – the universe! From the infinite-to-the-infinitesimal, when it comes to actual material stuff, the universe truly is much ado about nothing.
What’s the deal with the universe? That’s a question comedian Jerry Seinfeld might well posit in one of his stand-up performances. It came from nothing, it goes back to nothing and in between the coming and going there’s a whole lot of nothing. Seinfeld became immortalized by the eponymous sitcom that famously billed itself as “a show about nothing.” Of course, from that show about nothing sprang a cornucopia of expressions that have become part of the lingua franca – re-gifter, sideler, close-talker, man-hands, master-of-my-own-domain, yada, yada yada. And out of the nothingness from which the universe sprang we got – well – the universe! From the infinite-to-the-infinitesimal, when it comes to actual material stuff, the universe truly is much ado about nothing.
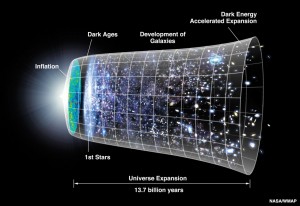
From a good observational point on a clear starry night take a look up into the sky and consider all those billions and billions of stars that so entranced Carl Sagan. Then consider the following. Collectively, all those billions and billions of stars account for about five-percent of the composition of the universe. Through observation of gravitational effects, we know there’s another 25-percent of the universe made up of material stuff we are not yet able to “see” or detect and we have dubbed this invisible stuff as “dark matter.” As for the rest of outer space, that great black cosmos, when it comes to stuff, the universe has got nothing.
But it gets worse. If you paid any attention at all in your high school science classes – and I do mean any attention – you know that all us and the world we live are made up of atoms, the smallest bits of matter that have chemical properties. And if you paid slightly more attention then you know that atoms consist of a central nucleus made up of one or more positively-charged protons and possibly one or more charge-less neutrons, and that this nucleus is orbited by negatively charged electrons. Even if you did not know that before, now you do. What high school science classes seldom make clear is how much nothing there is inside of atoms. Let’s take a look at the simplest and smallest type of atom – hydrogen. Its nucleus consists of a single proton that is orbited by a single electron. If you were to create a scale-model of a hydrogen atom, and you personally represented the proton in the nucleus of that hydrogen atom, then your electron would be represented by a pea and it would be located approximately 13 miles away from you. In between you and your electron would be nothing.
Think about a time you met someone and came away thinking to yourself what an empty person that was. You were spot-on! But be careful about throwing bricks from your glass house. The physicist Brian Greene once calculated that if all the empty space in the atoms that make up a human body were removed – that is the space between the nucleus and the electrons of those atoms – every human being on the planet today could fit inside a teacup with room to spare. I think we can all agree that’s a lot of nothing. And don’t even get me started on all the nothing inside protons and neutrons, which are essentially hollow bags consisting of three quarks and (more than half) empty space.
So much empty space begs the question as to why we aren’t like ghosts, spectral beings able to pass through the so-called solid objects in what is itself essentially a phantom world? To answer that question we must turn to quantum mechanics, but don’t worry, there’s no math involved and there won’t be a quiz. First, the picture of the universe inside the atom that most high school science classes present is one in which a small moon-like electron orbits a larger planet-like nucleus. This simplistic picture is shown to avoid quantum mechanics for reasons that will soon become obvious.
The universe inside the atom is not at like the macroscopic universe that we and our planet move through. An electron is a fermion, a particle of matter that also acts as if it were a wave. When we talk about an electron’s orbit, we’re talking about a discrete region of space away from the nucleus that is occupied by that wave. Because of the negative electrical charge carried in that wave, no other electron can occupy that same orbit at the same time. The rule is called the Pauli Exclusion Principle after Wolfgang Pauli, the Austrian physicist who discovered it in 1925. This discrete region of space occupied by an electron wave is defined by its energy (which can have an upper and lower limit) and by the attraction between the negative electrical charge of the electron and the positive charge of the nucleus.
Rather than one or more moons orbiting a planet, the picture inside an atom is more akin to a nucleus with a staircase made up of energy steps, each which may be occupied by a single electron. Under the rules of quantum mechanics, for any given energy step, an electron can physically be anywhere and everywhere on that step at once. Even if that doesn’t make sense from the macroscopic world our senses are engineered to perceive, the important thing to know is that no other electron can be on that step except for that one electron. Hence that energy step for that electron in that atom and every energy step for every electron in every atom in the universe is as “solid” as the earth beneath your feet.
This brings us back to the original question: What’s the deal with the universe? Is it really about nothing? Of course not. There’s something there alright and it’s HUGE. Before talking about that huge something I would like to refer you back to an earlier blog in which I discussed the meaning of the word “theory” and how scientists and the general public use the word in two very different senses. When most of us talk about “empty” space we mean that it is void of matter, which is defined as anything that has mass and takes up physical space. In science, however, thanks to Albert Einstein, we know that mass and energy are interchangeable, two sides of the same coin. So when we say that matter, both the matter we can see and dark matter comprise about 30-percent of the space in the universe, the rest of it is not actually empty but filled with energy.
Some of this energy, such as the energy produced from the thermonuclear burning of the sun and other stars, is intertwined with matter, but the energy that makes up the remaining 70-percent of the universe is a mystery that has been dubbed “dark energy.” This so-called dark energy acts as a sort of anti-gravitational force that accelerates the rate at which the space of our universe is expanding. The discovery of dark energy was announced at the start of 1998 by two teams, the Supernova Cosmology Project head by Saul Perlmutter of the Lawrence Berkeley National Laboratory (my day-time employer) and High-z Supernova Search Team led by Brian Schmidt and Adam Riess. The three men shared the 2011 Nobel Prize in Physics for this discovery. No one knows what dark energy is but an awful lot of scientists are doing their best to figure it out because the fate of the universe hangs in the balance. But that’s another story.
